TECHNOLOGY

GenomeCare possesses proprietary AI model establishment technology, verified through patents and peer-reviewed publications, that analyzes cfDNA sequencing data obtained from maternal blood to identify fetal chromosomal abnormalities with high sensitivity and specificity.
For model training, not only actual normal datasets but also synthetic datasets derived from them were utilized. To this end, GenomeCare developed and validated technology capable of generating synthetic data with 99.99% similarity to real data. By combining traditional machine learning methods with state-of-the-art algorithms, including artificial neural networks, the company ensured both predictive performance and model stability.
Through this approach, GenomeCare provides next-generation, AI model–based services in the field of non-invasive prenatal testing (NIPT), capable of detecting autosomal aneuploidies, sex chromosome aneuploidies, and even fine-scale copy number variations with high precision.
In addition, as a leader in NIPT technology, GenomeCare is actively developing intelligent systems and decision-support technologies that utilize large language models (LLMs) across multiple areas—including service chatbots, automated result interpretation, and report generation—strengthening its expertise and competitiveness in AI-driven healthcare.
To dramatically improve the precision of NIPT, GenomeCare has developed proprietary synthetic data generation technology that creates large volumes of virtual negative and positive datasets, which are then used to train AI models.
This synthetic data generation technology produces datasets through two distinct methods.
By establishing NGS analysis methods and applying SNP prediction models, GenomeCare developed algorithms to design synthetic datasets in silico, enabling the large-scale generation of expanded data.
By securing quality-control material data and designing new methods for generating reference materials, GenomeCare produces expanded datasets in vitro.
Validation of Synthetic Data
To confirm similarity between synthetic and real datasets, the frequency distribution of DNA fragment sizes was compared, and correlation coefficients were calculated. This verified a similarity of over 99.99%.
Positive quality-control specimens from real disease cases were obtained, and model prediction results were generated to indirectly validate the synthetic datasets.
Based on synthetic data technology validated for similarity to real clinical data and biological reliability, GenomeCare has achieved major technical milestones, including the development of synthetic data algorithms for detecting sex chromosome aneuploidies and copy number variations, as well as the creation of high-precision predictive models utilizing diverse AI algorithms.
This technology overcomes the limitations of conventional NIPT, dramatically improving both positive predictive value (PPV) and sensitivity, and enabling the prediction of a wider range of disease types.
Synthetic data generation technology is now positioned as a core foundation of NIPT, reducing the need for costly confirmatory tests such as amniocentesis while ensuring both accuracy and cost-effectiveness.
Published Patent: 10-2023-0157204
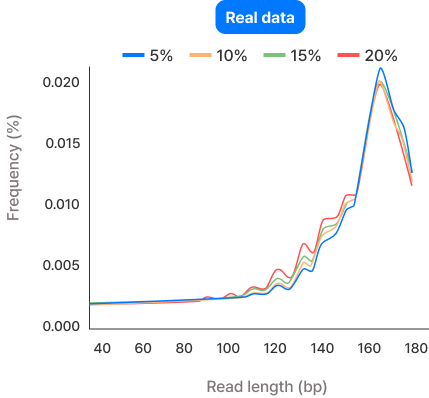
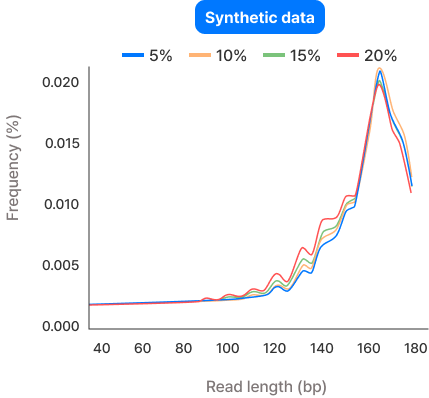
| Fetal DNA fraction | Correlation |
|---|---|
| 5% | 99.99% |
| 10% | 99.99% |
| 15% | 99.99% |
| 20% | 99.99% |

The company informs customers through the Privacy Policy about how the personal information provided is used and what measures are taken to protect personal information.
- Identifying individuals, verifying real names, confirming intent to sign up, and ensuring age-restricted service usage based on service use.
- Fulfilling contracts related to service provision, such as billing for services provided, delivering content, purchasing and processing payments, and dispatching items or billing statements.
- Delivering notices, ensuring communication for handling complaints, and ensuring accurate delivery information for item shipments.
- Providing information on new services and offering personalized services.
- Facilitating smooth provision of high-quality services.
- Name, company name, email, address, contact number, mobile phone number, inquiry details, and other optional items.
- As a general rule, personal information is destroyed immediately once the purpose for its collection or provision has been fulfilled.
- However, for the purpose of ensuring smooth service consultations, the content of the consultation may be retained for 3 months after completion. In cases where other laws, such as the Act on the Consumer Protection in Electronic Commerce, require preservation, the information may be retained for a specified period.
The company, as a general rule, destroys personal information without delay after the purpose of collection and use has been fulfilled. Information entered for membership or other purposes is transferred to a separate database (or, in the case of paper records, to a separate file) and stored for a certain period according to internal policies and relevant laws before being destroyed. Personal information transferred to a separate database is not used for any other purpose unless required by law. Personal information stored in electronic file formats is deleted using technical methods that prevent the recovery of the records.

The collection of email addresses posted on this website using email collection programs or other technical devices is prohibited, and violators may be punished under the Act on Promotion of Information and Communications Network Utilization and Information Protection.