SERVICE

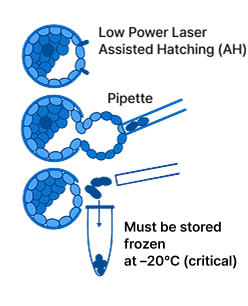
Preimplantation Genetic Testing (PGT) involves retrieving a few cells from embryos on day 3 or days 5–7 after in vitro fertilization (IVF). The DNA is amplified and analyzed using next-generation sequencing (NGS) to detect chromosomal abnormalities.
By selecting and transferring genetically normal embryos through PGT, the chances of achieving a successful pregnancy with IVF can be significantly improved.

High accuracy

Minimal sample required

High sensitivity

Faster turnaround time

As of the first half of 2024, available at more than 52 hospitals nationwide
Day 3 embryo (Blastomere)
1~2 cells
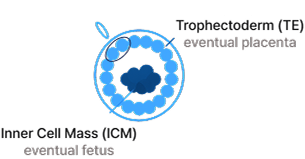
Day 5–7 embryo (Trophectoderm)
5–10 cells
GenoBro
Dedicated PCR tube
For an embryo created through IVF to successfully implant and sustain a pregnancy, not only its appearance but also its chromosomes must be normal.
Embryos with chromosomal abnormalities are more likely to fail implantation or cause recurrent miscarriage. In addition, as maternal age increases, the proportion of genetically normal embryos decreases.
To improve implantation rates and reduce the risk of miscarriage, it is essential to select chromosomally normal embryos for transfer through preimplantation testing.
Embryo biopsy (embryo cells)

Secure delivery by a certified courier service

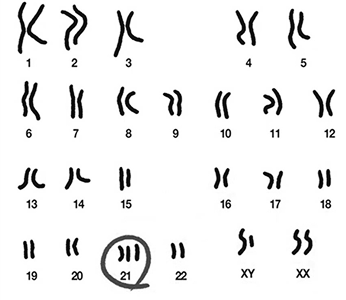
Next-generation sequencing (NGS) analysis

Detection of chromosomal abnormalities in embryos
| Test name | GenoBro (Preimplantation Genetic Testing, PGT) |
|---|---|
| Method | Next-generation sequencing (NGS) (NGS, Next Generation Sequencing) |
| Test items | PGT-A: Screening for numerical chromosomal abnormalities in embryos PGT-SR: Screening for structural chromosomal abnormalities in embryos |
| Testing period | Day 3 or Days 5–7 of in vitro embryo culture |
| Specimen information | Day 3 embryo: blastomere cells (partial sample) Days 5–7 embryo: trophectoderm cells (partial sample) |
| Submission deadline | By 12:00 p.m. (noon) |
| Turnaround time | Next business day (if received before 12:00 p.m.) Two business days later (if received after 12:00 p.m.) |

The company informs customers through the Privacy Policy about how the personal information provided is used and what measures are taken to protect personal information.
- Identifying individuals, verifying real names, confirming intent to sign up, and ensuring age-restricted service usage based on service use.
- Fulfilling contracts related to service provision, such as billing for services provided, delivering content, purchasing and processing payments, and dispatching items or billing statements.
- Delivering notices, ensuring communication for handling complaints, and ensuring accurate delivery information for item shipments.
- Providing information on new services and offering personalized services.
- Facilitating smooth provision of high-quality services.
- Name, company name, email, address, contact number, mobile phone number, inquiry details, and other optional items.
- As a general rule, personal information is destroyed immediately once the purpose for its collection or provision has been fulfilled.
- However, for the purpose of ensuring smooth service consultations, the content of the consultation may be retained for 3 months after completion. In cases where other laws, such as the Act on the Consumer Protection in Electronic Commerce, require preservation, the information may be retained for a specified period.
The company, as a general rule, destroys personal information without delay after the purpose of collection and use has been fulfilled. Information entered for membership or other purposes is transferred to a separate database (or, in the case of paper records, to a separate file) and stored for a certain period according to internal policies and relevant laws before being destroyed. Personal information transferred to a separate database is not used for any other purpose unless required by law. Personal information stored in electronic file formats is deleted using technical methods that prevent the recovery of the records.

The collection of email addresses posted on this website using email collection programs or other technical devices is prohibited, and violators may be punished under the Act on Promotion of Information and Communications Network Utilization and Information Protection.